Archives
Chiral Molecule Scattering Experiments
|
So, can we observe electron optical activity? First of all, to this date, experiments in this field have primarily been concerned with electron circular dichroism rather than electron optical rotation. Circular dichroism measurements are simpler because they require only the detection of intensity variations, whereas rotation experiments require measurements of very small changes in electron polarization. Also, circular dichroism measurements are historically prominent because of the discovery of parity violation in β-decay in 1956 and the subsequent theories by Vester and Ulbricht for the origin of biological homochirality by the interaction of β-rays with primordial chiral molecules. Therefore, let's focus on the observation of electron circular dichroism. As mentioned earlier, when measuring an ECD signal, it is common to modulate the polarization of the electron beam and scatter the beam from molecules of fixed handedness. This polarization modulation leads to a modulation in the transmitted electron beam current if the target is dichroic. Such an intensity modulation is usually expressed as an asymmetry defined as 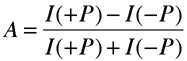
I(+P) is the transmitted intensity of right-handed electrons, and I(-P) refers to that for left-handed electrons. The electron current is collected by a Faraday cup and is measured by an electrometer. Here is a schematic of the data collection technique: 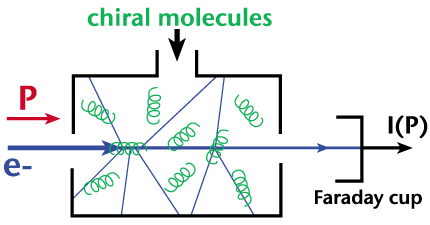
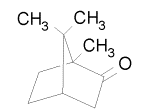
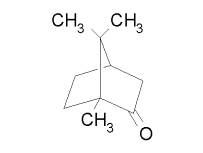
In 1985, Campbell and Farago made the first attempt to see ECD. They used camphor vapor as their target, and the energy of their electron beam was 5 eV with a polarization of 28%. The asymmetry they observed was about 10-3. While this result seemed to be the first observation of ECD, theorists were skeptical. Camphor, C10H16O, contains no heavy atoms, and most calculations revealed that the ECD asymmetry would not be seen unless a high-Z atom was present. Here are the enantiomers of camphor:
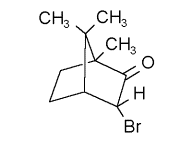
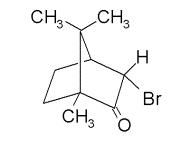
This discrepancy led Kessler's group (Mayer et al) in Munster, Germany as well as our group (Trantham et al) at UNL to attempt a verification of Campbell and Farago's results. As seen below, both groups observed zero asymmetry in camphor to an accuracy better than 10-4 for electron energies from ~ 0 to 10 eV. In 1995, Mayer and Kessler were able to see nonzero asymmetries for targets with heavier nuclei. Specifically, they used bromocamphor (Z=70)
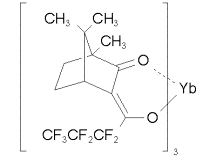
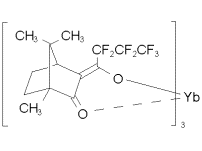
and Yb(hfc)3 (an NMR shift reagent)
Their asymmetry spectra showed that both of these compounds have maximum asymmetries of ~ 2 x 10-4. However, this result is puzzling because the bromine atom in bromocamphor is much lighter than the ytterbium atom in Yb(hfc)3. Theory predicts the asymmetry to increase as Z2. So perhaps the molecular structure plays a role just as significant as that of atomic mass. In Yb(hfc)3, the Yb atom is not at the chiral center of the molecule. To specifically test the Z dependence of the asymmetry, the Munster group then took spectra of [X(hfc)3] where X = Pr, Eu, Er, and Yb. This series has a 40% variation in Z2 and should thus have yielded an obvious range of asymmetry values. However, their data show that in all cases the asymmetries are of the same order of magnitude: ~ 1-2 x 10-4. |
|||||||||||||||||||||
|
Archived: January 2020 |