Archives
Chiral Molecule Scattering Experiments
|
Chiral Molecules
How can molecules exhibit handedness? With regard to organic chemistry, chiral molecules commonly comprise a carbon atom attached to four different substituents. The substituents can be either atoms or groups of atoms, but each must differ from the other three for the carbon atom to be a chiral center (stereocenter). Here is a sketch of a basic chiral molecule: 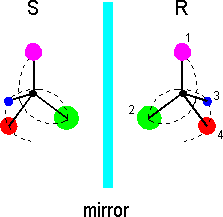
All the substituents are a different color, and the molecule's handedness can be defined by drawing a spiral through the substituents according to the Cahn-Ingold-Prelog rules [see any organic chemistry text]. Each group attached to the stereocenter is given a priority according to its atomic number (mass). Higher priority (1) is given to the substituents with higher atomic numbers, and the spiral thus begins with the lowest atomic number (4). If isotopes of the same atom are involved, the more massive ones take higher priority. If two identical atoms are attached to the chiral center, the next atoms in the two substituents are examined. Moving outward from the chiral center, priority is given at the first point where the substituents differ.
The left- and right-hand configurations of chiral molecules are dubbed enantiomers. Left-handed enantiomers are given the label S - for sinister in Latin - and the right-handed enantiomers are labeled R, for rectus. These labels indicate the absolute configuration of the molecule; i.e., they refer to the actual orientation in space of the substituents around the stereocenter. By placing R or S in front of the name of a molecule, its configuration can described without the aid of a three dimensional picture. Note: Many chiral molecules have more than one stereocenter. Also, the stereo center of a chiral molecule need not be a carbon atom. Some tetravalent phosphorous and trivalent sulphur compounds are also chiral, for instance. Furthermore, some chiral molecules lack a stereocenter that has at least four substituents. However, for brevity, we will not discuss these types of systems here.
For further information about stereochemistry see the following links:
Shockwave tutorials of organic chemistry |
|||
|
|
|||
|
Archived: January 2020 |


