Projects
Ultrafast Nanotip Emitters of Spin-Polarized Electrons
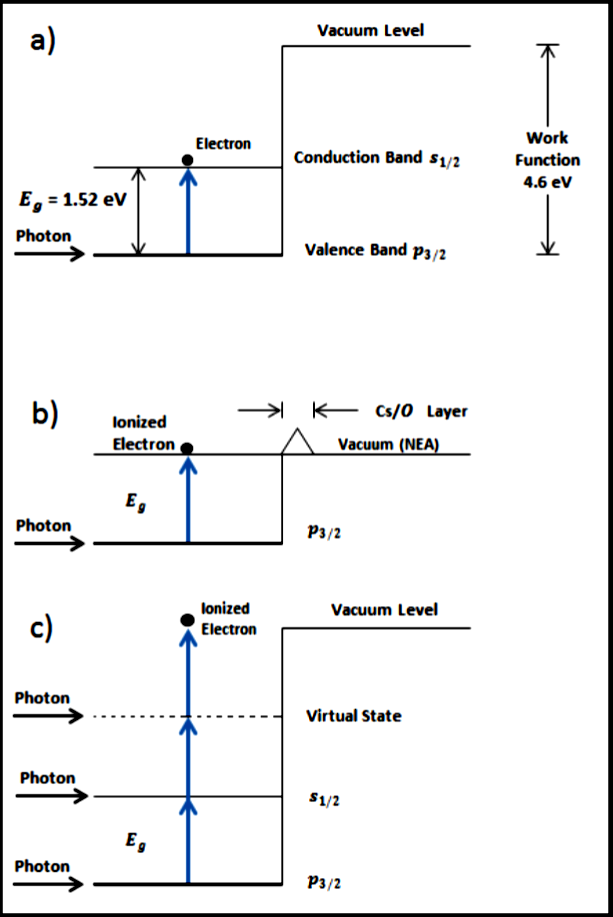
Figure 1: a) Energy band structure of positive electron affinity GaAs and single-photon absorption. b) GaAs with Cs and O layers creates a negative electron affinity. c) Multi-photon optical pumping to achieve ionization. Image taken from M. LeDoux, et. al, Photoemission by Multi-Photon Absorption in GaAs. The production of spin-polarized electrons is of paramount importance in the world of atomic, molecular, and optical physics. The Fast Spin-Polarized Electron Source experiment in the Gay Research Group is a novel method of producing spin-polarized electrons from a gallium arsenide tip-source by illuminating it with a femtosecond laser pulse. This method is an improvement on the more traditional method, which relied on coating the surface of a GaAs source with a layer of cesium and oxygen in order to create a surface with a negative electron affinity. Previous studies of this method have shown that it is possible to produce spin-polarized electrons of 13% polarization. Here we will go into more detail on the method of fast spin-polarized electron emission. Gallium arsenide in the bulk has a gap between the conduction and valence bands of about 1.42 eV. Excitation of electrons in the valence level to the conduction band would correspond to an approximately 800 nm wavelength laser. However, the conduction band is about 3 eV below that of the vacuum level, meaning that clean GaAs has a positive electron affinity. Production of an electron beam by photoemission cannot be obtained from bulk GaAs unless steps are taken to modify it to create negative electron affinity (NEA) GaAs. In practice, NEA GaAs has been created by depositing atomically thin layers of Cs and O onto the GaAs crystal. A surface of clean GaAs that has been coated with Cs and O will sufficiently reduce the vacuum level to below the conduction band. Thus a continuous wave laser of near-band-gap energy can photo-emit electrons. In an ultrahigh vacuum environment, NEA GaAs can be used to produce spin-polarized electrons. When circularly-polarized light with sufficient energy is used to illuminate NEA GaAs, some photo-emitted electrons will be imparted with angular momentum from the incoming photons. The resulting electron beam is said to be spin-polarized, to a certain degree. We characterize the polarization of the beam as  where N↑ and N↓ refer to the number of electrons with spin-up and spin-down angular momentum with respect to an arbitrary axis. Traditional NEA GaAs photoemission has, in practice, produced beam polarizations of 25–35%. 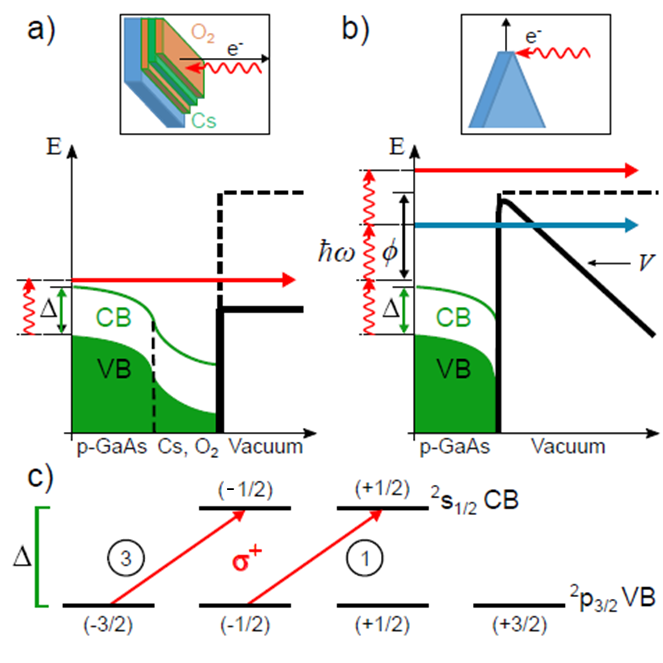
Figure 2: GaAs energy levels for (a) NEA bulk surfaces and (b) a non-NEA shard apex. The diagrams indicate bending of both the valence band (VB) and conduction band (CB) at the surface due to heavy p-doping. (a) The vacuum energy (dashed black line), is lowered (solid black line) due to the dposition of alternating layers of Cs and O2 (top insert). Electron emission from the NEA surface proceeds by the absorption of a single photon with energy that exceeds the band Δ of the bulk. (b) Multiphoton emission from an uncoated, non-NEA GaAs shard apex. (c) Allowed transitions at the GaAs Γ-point for absorption of right-hand circularly-polarized light by Zeeman (mj) sublevels. Selection rules (Δmj = +1) and the relative line strengths (indicated in circles) yield a nascent conduction-band electron polarization of (3 − 1)/(3 + 1) = 50% for valence-conduction band resonant transitions. Image source: Brunkow et.al. Femtosecond-Laser-Induced Spin-Polarized Electron Emission from a GaAs Tip. APL (2019). The Fast Spin-Polarized Electron Source project is exploring a different method of producing electrons from GaAs through a process known as multi-photon emission. This approaches the problem of a positive electron affinity from another perspective. Rather than treating the surface of bulk GaAs with other elements in an effort to lower the vacuum level, electrons are excited to the energy level necessary for emission (> 3 eV above the conduction band level) by optically pumping the electrons. This is achieved using a femtosecond, pulsed laser. Temporally separated—but closely spaced—pulses allow for a multi-staged process of imparting the electrons with energy. Normally for the photoelectric effect of GaAs, one would send in continuous wave light. An electron would absorb the photon and be excited to the conduction band. Before the electron could absorb another photon, it would decay back down to the valence band. Using pulsed light avoids this problem by containing a much higher density of photons in a given amount of time than continuous wave light. The electrons absorb one photon and are excited to the conduction band. Before they can decay down to the valence band, they quickly absorb another photon and are excited to a virtual state between the conduction band and vacuum. The electrons can then absorb a third photon and are excited to the vacuum level or tunnel through the potential into vacuum. Through this multiphoton process we can achieve a high number of emitted electrons without the need of a low electron affinity. The polarization of light governs the selection rules for the polarization of our emitted electrons, see Fig. 2. As stated above, this method has shown in the past to produce electron beams of about 13% polarization. The polarization measurement of our electrons is done through standard Mott scattering with a gold target. Our detectors lie at a scattering angle of 120 degrees where the polarization of scattered electrons is most distinct. |
Explore MorePapers
Posters
|
