Archives
A Compact, Efficient Mott Polarimeter
|
The electron has an intrinsic spin angular momentum. A beam of electrons is said to be “polarized” if their spins point, on average, in a specific direction. In 1929, the British physicist Sir Nevil Mott raised the question of whether or not the effects due to electron spin and polarization could be observed directly. He proposed that spin could be detected in a double scattering experiment in which a beam of high energy, unpolarized electrons are scattered from a high-Z nucleus. In 1942, Schull, et al. demonstrated a polarized scattering asymmetry that was in agreement with Mott’s calculated value. Today, the area of polarimetry has shifted towards measurement of electron polarization in other areas of physics. Principles of Mott ScatteringConsider a single electron scattering from a bare, high-Z nucleus. As the electron passes through the electric field of the nucleus, a magnetic field is produced in the reference frame of the electron. 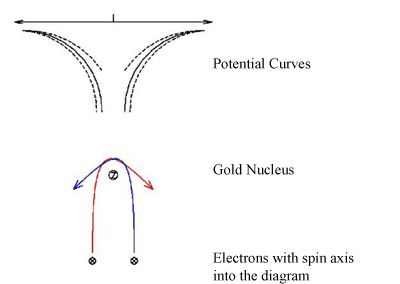
There are two forces that act on the electron as it approaches the Au nucleus, one due to the magnetic field and one due to the electric field. Because the electron has a spin angular momentum, the force exerted by the magnetic field depends on whether the electron scatters to the left or right. If the potential scattering curves are plotted for the electron, the curves become skewed one way or the other depending on the electron’s spin. This leads to an asymmetry in the left/right scattering probabilities: 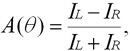
where IL and IR are the currents detected in the left and right channels of the polarimeter. Another important parameter in discussing Mott polarimeters is the figure of merit: 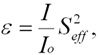
where Io is the current entering the polarimeter, I is the total scattered current, and Seff2 is the analyzing power of the apparatus: 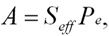
where Pe is the degree of electron polarization. Why Build a New Mott Polarimeter? Apparatus 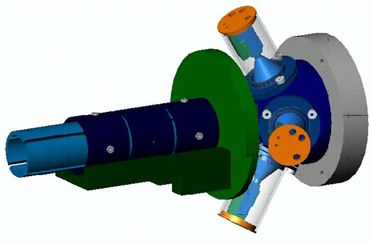
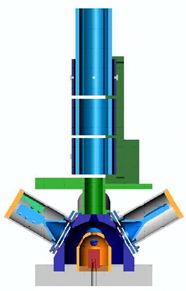
The electrons next enter the target chamber. The chamber consists of a cylindrical target within a polished stainless steel hemisphere. A common material used for the high-Z nuclei target is gold. Careful consideration must also be employed in choosing the material. Low-Z nuclei help minimize unwanted scattering, so aluminum was chosen. Scattered electrons then exit the target chamber and are collected in the detectors. The target chamber is shown in detail below. The transport system has been removed. The target is shown in red and the inner hemisphere in orange. 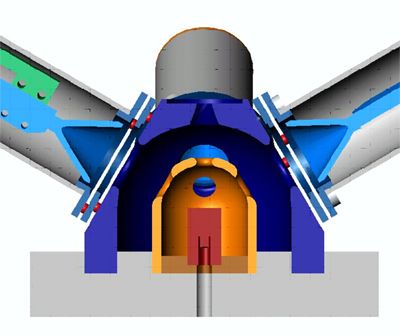
Results 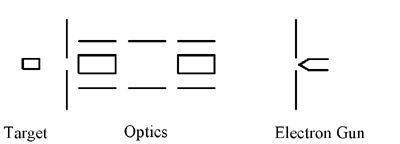
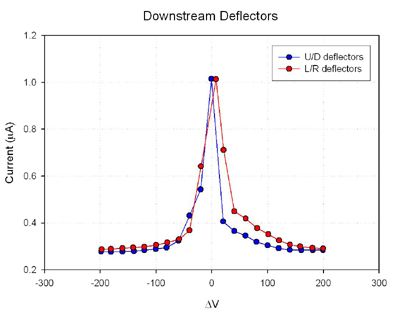
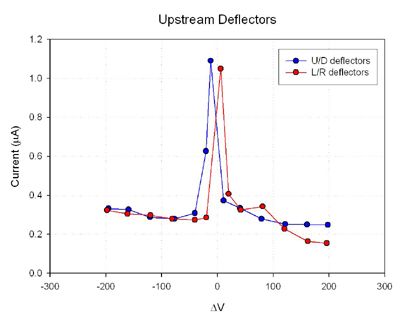
Future Experiments References
This project was funded by NSF Grant No. PHY-0099363 and UCARE |
|
Archived: January 2020 |
